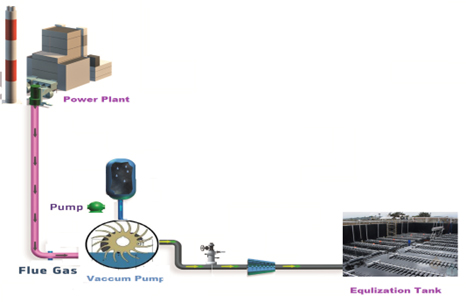
Stack gas CO2 Alkaline effluent neutralization
Compared to mineral acid carbon dioxide neutralizes alkaline wastewater at very low consumption. The well balance self adjusting properties of CO2 does not led to increase the pH of water, therefore this eliminating the operator attention in the plant.
CO2 react with Alkalinity of Wastewater that buffering the pH changes. Alkalinity is equal to the concentration of bicarbonate HCO3–, carbonate CO32- or hydroxyl OH- ions.
Due to the mass transfer limitation, small portion of CO2 is converted into carbonic acid by this reactions
Carbonic acid is a mild acid present in water as ions H+ and HCO3–, which are highly reactive. They will immediately react with ions responsible for alkalinity of water.
NWD offers complete design and engineering of stack gas CO utilization for alkaline pH neutralization to reduce the acidic waste. We have successfully executed couple of units in Textile wastewater neutralization.
Application Paper mill wastewater, Textile effluent, cement industry and paint and coating.